Regulatory science provides the Medicines Evaluation Board (MEB) with up-to-date data, knowledge and expertise based on current scientific insights, for optimal evaluation and marketing authorisation of medicinal products and novel foods.
The studies that support the MEB's regulatory process are very diverse. The MEB Regulatory Science programme presents several studies as a Poster Pitch and also some Portraits.
Science Pitches & ProfilesHere you find an overview of all the previous MEB's science poster pitches and profiles of the PhDs, starting in 2012.
2018

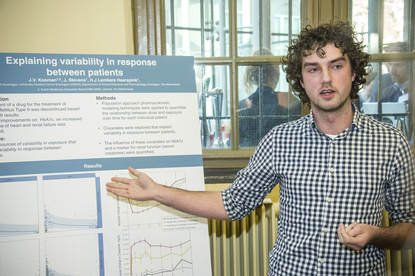
Science Pitch Jeroen Koomen: Explaining variability in response between patients.
(The MEB's logo. On-screen title: Poster pitch by J.V.Koomen, MSc. On-screen text: Explaining variability in response between patients.)
MAN'S VOICE: My PhD project is about explaining variability
in response between patients,
and mainly in patients with type 2 diabetes.
So, in this particular project, I looked at a drug that was intended
for a treatment for type 2 diabetes,
but was failed after the outcome of a large Phase 3 study.
And in the study, they saw a lowering of HbA1c,
which is a marker for glucose lowering,
but they also saw an increased incidence of heart failure and renal failure.
Our objective in this project was
to quantify the variability in exposure between patients,
to look at the response between patients.
So, we used the population pharmacokinetic approach to identify covariates
that could explain variability in exposure,
and that is displayed in this graph over here.
In this graph, you can see the observations of the patients and the modal overlay.
And as you can see, there are four graphs,
so we identified food intake and comedication as covariates
in a population pharmacokinetic model.
So, we then looked at the pharmacodynamic response on HbA1c
and on serum creatinine, which is a marker for renal function.
And as you can see, the placebo groups do not differ from each other,
but patients who used the study drug with comedication
had an additional lowering of HbA1c and an additional decrease in renal function.
So, in conclusion,
we identified comedication and food intake as covariates
that could explain variability in response between patients.
And we showed that the comedication
was the cause for an additional lowering of HbA1c,
however at the expense of an additional worsening of the renal function.
And questions remain: should we, as a regulator, ask companies to identify causes
that could explain variability in response between patients?
(The MEB's logo, next to: Medicines Evaluation Board.)

Science pitch Carla Jonker: What to gain on registries?
(The MEB's logo. On-screen title: Poster pitch by C. Jonker, MSc. On-screen text: What to gain from registries?)
WOMAN'S VOICE: My PhD project is about registries
and the role of registries in the regulatory decision process.
For all new drugs approved in the period January 2007 up to December 2010,
we reviewed the European Public Assessment Reports, the EPARS,
that are published on the website of the EMA, the European Medicines Agency.
We looked for the word registry.
A registry is an organised system that collects data using observational methods
on a specific outcome in a population
that is defined either by disease or by exposure to a drug.
Our goal was to look into specific characteristics of the drugs,
to see if they could predict if a registry was available.
We looked into drug characteristics.
For example, the level of innovation.
This is an algorithm based on the availability of a treatment
for a certain disease or the therapeutic effect.
And we looked into procedural characteristics, like:
does a drug have an orphan status?
In my study, we found that there were 160 drugs approved,
and for one-third of them, a registry was requested.
It was one up to six.
So, we obtained a total of 73 registries.
Two-thirds of them were disease-based, and one-third, product registries.
The main reason for requesting a registry was because of safety,
for example to follow up on a safety concern,
or to collect data on women who are pregnant,
and in some cases we would like to know the real-world efficacy data.
The level of innovation and the orphan status
were positively related to having a registry in a dossier.
So for innovative drugs and orphan drugs,
a registry is important to collect data, post-approval.
(The MEB's logo, next to: Medicines Evaluation Board.)

Pieter Glerum presents his study on "Robustness of the conclusion of bioequivalence; a non-parametric comparison."
(The MEB logo, next to: Poster Pitch by P.J. Glerum: Robustness of the conclusion of bioequivalence; a non-parametric comparison.)
SILENCE
We're performing this research because there is a lot of public debate
about efficacy, safety and quality of generic medicines.
And for the registration of generic medicines,
bioequivalence needs to be proven.
And we are challenging the concept of bioequivalence.
Is that still the best way to register generics?
The research is still ongoing. We're not sure yet what this means for the patient.
Basically, there are two possible outcomes for this research.
First would be that this supports the current policy and regulations
for the registration of generics.
Second would be that we would indicate some flaws
in the system and regulatory requirements.
So then, we're challenged to look at improvements of the system.
Either way, the patient or the healthcare professional can be ensured
that we challenge the concept of bioequivalence and make sure
that the best possible way of registration of generics is in practice.
To get to these results, uh...
together with University Maastricht and Radboud University,
we started a collaboration with the University of Southern California
to learn about pharmacokinetic modelling.
And we applied this mathematical modelling to a bioequivalence study
with four different formulations of Gabapentin,
of which one is the originator, and there are three generics.
Now, if we look at the second picture on the poster,
this picture represents actually
the estimated absorption constant values per subject, colour-coded per formulation.
So, if this were the result of the research, there would actually be
an altered absorption profile estimated for one of the formulations.
But again, this is preliminary research, and the research is still ongoing.
(The MEB logo appears on a white background.)
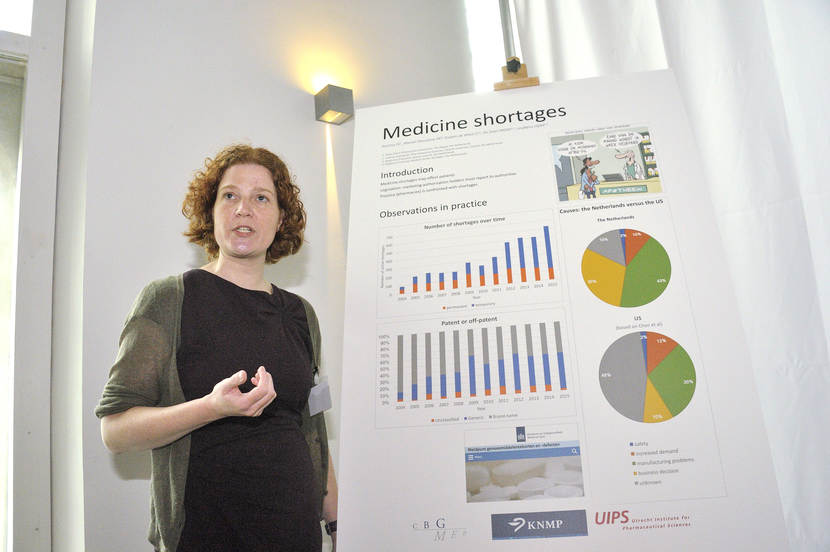
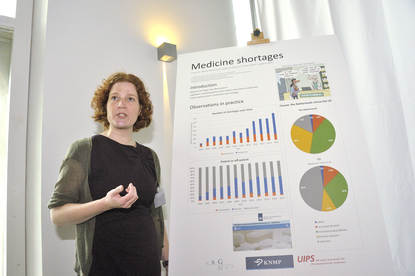
Doerine Postma presents her study on medicine shortages.
(The MEB logo, next to: Poster Pitch by D.J. Postma: Medicine shortages.)
SILENCE
The motivation for this study was
that medicine shortages have an impact on patients.
Patients can have to switch to another label,
to another active ingredient or the active ingredient is totally absent.
Most studies are now based on surveys or on anecdotes, but not on hard data,
and this study is.
And by determining risk factors and understanding medicine shortages,
we can help to lessen the impact for patients.
The results, up until now, are based on one data set.
It is based on the reporting system from the Royal Dutch Pharmacists Association,
where pharmacies voluntarily report their shortages.
We're going to have a closer look at the data from authorities
where the marketing authorisation holders mandatorily report their shortages.
The results are now mainly based on observations from practice.
When we look at the number of shortages,
we see an increase especially in temporary shortages.
The number of permanent shortages is stable.
Looking at the difference between patented and off-patented medicine,
we see an increase of the patented medicine up until 2009,
and then the share of patented and off-patented medicine
is about 50 percent each.
Looking at the causes of medicine shortages,
we compared our results to the U.S.
And we see a difference in manufacturing problems,
and also a difference in business decisions.
It is hard to draw hard conclusions,
because a very large part of the causes are unknown in the US.
Since 1 January 2017,
the Medicine shortages and defects notification centre has been introduced.
Marketing authorisation holders have to report their shortages to the authority,
leading to sooner awareness of the shortages in practice.
(The MEB logo appears on a white background.)

Remy Francisca presents his study on the impact of the 2012 Pharmacovigilance legislation on required additional risk minimisation measures.
The MEB logo, next to: Poster Pitch by R.D.C. Francisca, MD: Impact of the 2012 Pharmacovigilance legislation on required additional risk minimisation measures.)
SILENCE
What the motivation was for this research
is that we wanted to see what the effect was of a law revision
which came into force in July of 2012 on additional risk minimisation measures.
These are measures which can be employed to minimise the risks
associated with use of medicinal products that are extra.
These are on top of measures that are always employed for all drugs
and should be used sparingly in exceptional circumstances.
But previous research has shown
that one in three drugs, up to 2009, got these measures,
and we wanted to see what the effect of the law on this percentage was.
We used publicly available data,
which is available on the website of the European Medicines Agency,
where they publish the European Public Assessment Reports.
These EPARs contain information on the initial assessment of the drugs
that allowed them to be licensed.
They contain information on the marketing authorisation.
And they also contain information on the risk management plan,
which is what we needed, to get our data.
What the results of this research are, is that we found that before the law revision,
which is 2011 and 2010, 38 percent of the drugs were assigned additional measures,
and after the law revision you see that that percentage drops to 28 percent.
So, it's a 10 percent drop,
but when you look at it through statistical means, it's not significant.
So, it could still be sort of by chance that we find these results.
And what it means for clinical practice, and patients in general, is that,
when you look at the types of measures that can be employed which are extra,
you see that extra communication of risks to patients has increased.
So, we inform patients more often about what risks are associated
with the use of their drug.
(The MEB logo appears on a white background.)
2012 - 2016

- Taina Mattila: Bridging from drug registration trials to meaningful clinical evidence-the case of schizophrenia (7-7-2016/University of Amsterdam)

Science pitch Tessa Hulshof : Why small baseline differences matter in RCTs

Science pitch Marlies Kubbinga : Excipients and the bioavailability of oral medicines

Science pitch Charlotte de Wolf : Will in vitro assays replace laboratory animal tests?

Science pitch Yang Yu : Generic Interchangeability: Can we trust generic medicines?