When a child is sick, that child should receive the right medicine - that much is obvious
Professor Saskia De Wildt has been working on improvements in the field of paediatric medicines for the past 20 years. But as a professor, paediatric intensivist and clinical pharmacologist working at Radboud UMC in Nijmegen and Erasmus MC in Rotterdam, she also knows that there is still a lot of work to be done. That is why she is also the director of the Kinderformularium ("Children's Formulary") and coordinates the Dutch trial network PEDMED-NL as well as the European network of experts, which are both part of the pan-European network for medicine trials among children, Conect4Children.

Kinderformularium
The Kinderformularium provides independent information about medicine use in children.
"I hope that in 20-30 years we will have good data for all children’s medicines. And that we can all agree on that data. And that we can get there in a child-friendly way, minimising the burden and risk. I also hope that one day the Kinderformularium won’t be needed any more. So we can provide children with medication that we know is safe and effective. Including medicines that are already on the market and that we have been using for a long time. It should be obvious that a sick child should receive the right medicine, in the most appropriate way for that child. That’s the goal we have to aim at," explains Saskia.
In this video Saskia de Wildt shares here view regarding the MEB and paediatric drug development.
Saskia de Wildt – Pediatrician and clinical pharmacologist RadboudUMC:
WOMAN's VOICE: It took quite long to get legislation to force
companies to study drugs in children...
because before that time we thought...
that just adjusting the dose for children
based on the adult dose would be enough.
But because incidents occurred
and children were actually harmed...
or children did not actually get effective therapy...
we started to realize
that this was not the right approach.
Then it took quite a bit of time, I think,
to also lobby governments...
that they had to put a legislation in place
to force companies to study drugs in children.
There is a difference
between drugs in children and in adults.
When you give a drug to a child,
the way it goes through the body is different.
Absorption may be different,
drug metabolism in the liver may be different...
and excretion, for example, in the kidneys.
Also, the biology of the child is different.
So, side effects may also be different,
because of this change in biology.
Even today, when I work in a pediatric
intensive care unit I prescribe drugs to children...
of which I do not know if they are
actually effective in that specific child...
or if they will be safe in that child.
My interest in this topic came
when I started my PhD...
where I started to do research
to understand how children's bodies are different.
Through this work I became
the director of the pediatric Dutch formulary.
Through my research and this work
I also got involved in European initiatives...
to improve our infrastructure
on how to perform drug studies in children.
Like the recent project we got funding for,
connect4children.
The key goal of connect4children is that in
5.5 years we will have a European-wide network...
that is able to perform effectively
and fast clinical trials in children...
that have been designed in a way
that they are as child-friendly...
and as professionally optimal as possible.
These initiatives are already generating results.
The c4c project in itself will provide results.
The goal is that in 5.5 years
we will have an infrastructure in Europe...
that's able to help design
and conduct clinical trials in children.
I myself see it more as a changing continuum
where we improve almost every single day.
I think this is the next step in this process...
that will really help us to get children
to receive effective and safe medicines.
I would be extremely excited if I could go
to my PICU, speak with parents and tell them...
that the drugs that we will be giving will be working
in their child and that they will be safe.
That they can look them in the eye
and be absolutely sure about this.
Title: Good medicines used better.
(The MEB's logo, next to: Medicines Evaluation Board.)
When she was training to become a doctor, she used to keep a notebook: "I would carry it around in my white coat. And every time I administered a medicine to a child that was not included in the Pharmacotherapeutic Compass or in the package leaflet, I noted down which dosage I had been given by a colleague. Every doctor had a notebook like that. But that meant that every doctor might prescribe a different dosage."
For a long time, it was thought that medication for children could be calculated simply by adjusting the adult dose according to the child’s weight. But there were a few cases where that method proved unreliable. "Because of those cases, we came to understand that we had to do things differently. It’s hard, because children are not just ‘small adults’ when it comes to designing and carrying out trials and research." In 2007, the law changed and since then new medicines that are in principle suitable for children must also be tested in children. But not all medicines have been studied thoroughly in children, even those that have been around for a long time. For half of the 750 medicines for children included in the Kinderformularium, the SmPC* is not compliant, says De Wildt. "Because the child indication has not been accepted. Or because the dose indicated is not accepted by us as doctors due to new data that has become available in the meantime. Not from the manufacturer, but from the academic research."
The Kinderformularium was established ten years ago, simply due to the need for better information. The Kinderformularium provides information about child dosages for 750 medicines. "We collate the best evidence and keep it up to date, and we’re always searching the literature for new information. After all, the benefit-risk analysis, which regulators like EMA and MEB ask for, may show that there is too little evidence, but in reality we’re already administering these medicines in children. Because if we avoided every medicine that was off-label for children, then we would end up undertreating some children, and that could even be fatal. So we have to do something."

Pharmacotherapy Improvement Cycle
De Wildt is the key figure within the Pharmacotherapy Improvement Cycle. The cycle is not only about improving medicines for children, but also improving and making information available about the dosing of children’s medicines. This is done through initiatives such as the European Paediatric Translational Research Infrastructure (EPTRI), which rapidly transfers and translates knowledge and innovative technology so that it can be used in clinical trials. And through clinical studies carried out as part of the Paediatric Clinical Research Infrastructure (PEDCRIN) and Conect4Children.
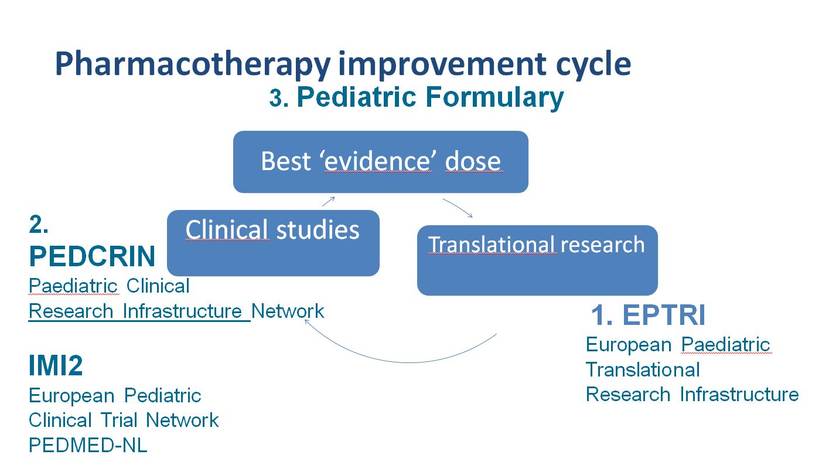
"What’s the right way to set up such a medicine trial in children and what do you need to know about the biology of children, about preparing a formulation - a drink or a pill - how do you measure the effect, which biomarker do you use? The EPTRI project aims to provide support infrastructure for research that can answer all the latest questions. For instance, you cannot start a clinical examination if you have not got these things clear first. If nobody develops measures that you can use to assess pain in children, for example, you’ll have to continue to use the adult method. But that’s just not suitable for a new-born baby. To come up with the right dosage for children, you also need to know how the gut and the liver change with age, and how absorption and metabolisation are affected as a result. Once these building blocks are clear, Conect4Children comes into the picture."
Coordination of studies in children by a European network
Since the legislation was amended, all manufacturers have been required to submit a plan for each new medicine that they are developing. Over a thousand plans have been approved. But in the end, changes have been implemented for fewer than 50 medicines, says De Wildt. "Many studies were unsuccessful. Due to their design: wrong outcome measure, too difficult, things that are not possible in children. The information was not implemented adequately in those studies. In adult cardiology and oncology, we have years of experience in conducting clinical studies. But for children's studies we have much less. And certain issues crop up regularly. For example, the question of consent is more complicated. And it is difficult to take blood from children, so you need specially trained staff. These factors are the same all across Europe. A network was set up in the UK 10 years ago. There was also an attempt in the Netherlands but that failed, as it did in other countries. We weren’t ready for it."
A few years ago, England launched an initiative to create a European network: Conect4Children (C4C). This is a partnership involving 33 academic centres and ten pharmaceutical companies from twenty different countries. "We want to receive applications at one international point in Europe and that is C4C. C4C enables the national network to ask: can you complete this study, what will it cost and are the centres able to do this?"
In May 2018, C4C received €140 million euros from the European Union and the pharmaceutical sector to set up a network, the purpose of which was ultimately to close the knowledge gap regarding the effects of medicines in children. "We were lucky to receive so much money. This is the largest grant ever for paediatric medicine. The 6 studies that are being carried out to test the network will be completed in 5 years. That will be the moment of truth, but I think the results are already noticeable. Simply because we’re already talking to each other at the European level now. There is more awareness, and we are going to be working together."
Bringing the right specialists and the right knowledge together
PEDMED-NL is the Dutch trial network that C4C works with. De Wildt is its coordinator: "We are thinking about what it should look like. All academic and general hospitals in the Netherlands are interested and they think it is important to make a success of it. With PEDMED-NL, we want to make all the information that there already is available more quickly. And when we receive an application for a study, we should be able to say within three days: 'Yes, we can do that, we have this many patients and this is the cost'. PEDMED-NL will also keep track of how many children's studies there are in the Netherlands. The aim is to increase this number by the end of the project.
Based at Radboud UMC, we will set up a European expert network, also within the framework of C4C. The aim is to help manufacturers and researchers to set up better children's studies with doctors, methodologists, and parents’ patients. We have various groups of experts, such as paediatric cardiologists, paediatric infectious disease specialists, statisticians, pharmacologists and Young Patient Advisory groups. These are young teenagers who come together to look at the study protocols and give their advice. We also have links with various patients’ parents associations. We want to bring the right specialists together with the right knowledge. We want to make study protocols more feasible and more innovative. And we want to include the innovations that are already underway in these studies."

Pioneering medicines research in children
De Wildt also studied clinical pharmacology. She was looking for a translational PhD study, and chose drug metabolism in children. She also wanted to make sure that her research generated new knowledge. "Back then in 1997, we were actually the first to research medicines in children - the pioneers." Her supervisor took her to international conferences and she met the people who were working on changing the legislation. After completing her PhD, she decided to become a clinical pharmacologist and became the first paediatric clinical pharmacologist in the Netherlands.
She was involved in the Kinderformularium right from the start. "I enjoyed exploring the wide field of paediatric pharmacology. And that really needed to be done." Now, De Wildt is also setting up a European Children's Formulary. The Dutch Kinderformularium is being translated for Germany and Norway and soon it will be in use there too.
Kinderformularium partnership and the MEB
"Currently there are 10 new medicines on the market for the same clinical picture, and they all have to meet the requirements for registration. But that’s not possible, because there just aren’t that many children. At C4C, we will bring together companies, experts, parents, the EMA and the MEB to discuss how we can do this. And whether it is actually necessary. And for medicines where it is very difficult to measure the effect, we want to look at what requirements should be set. I see a role for the MEB in doing that - thinking about how you can set up as many studies as possible, working in a child-friendly way and also using the data you already have as smartly as possible.
For the Kinderformularium, we sometimes have to depart from the SmPC. Our discussions with the MEB have helped us understand much better why they have decided not to register certain medicines. And at the same time, we have to consider whether we can look at the existing data with the MEB and the manufacturers. Maybe we can bring together expert opinions and data from other hospitals and include that in the package leaflet? Including a kind of disclaimer? Or including a kind of level of evidence? Working with the MEB we want to see how we can strike the best possible balance and whether we can develop new methods."
*The Summary of Product Characteristics of a medicine, which the manufacturer is required to provide.
Profile of prof. dr Saskia de Wildt, paediatric intensivist and clinical pharmacologist working at Radboud UMC in Nijmegen and Erasmus MC in Rotterdam