Regulatory science provides the Medicines Evaluation Board (MEB) with up-to-date data, knowledge and expertise based on current scientific insights, for optimal evaluation and marketing authorisation of medicinal products and novel foods.
The studies that support the MEB's regulatory process are very diverse. The MEB Regulatory Science programme presents several studies as a Poster Pitch.
Poster pitch by C. Jonker, MSc
Carla Jonker presents her study on "What to gain on registries?"
(The MEB's logo. On-screen title: Poster pitch by C. Jonker, MSc. On-screen text: What to gain from registries?)
WOMAN'S VOICE: My PhD project is about registries
and the role of registries in the regulatory decision process.
For all new drugs approved in the period January 2007 up to December 2010,
we reviewed the European Public Assessment Reports, the EPARS,
that are published on the website of the EMA, the European Medicines Agency.
We looked for the word registry.
A registry is an organised system that collects data using observational methods
on a specific outcome in a population
that is defined either by disease or by exposure to a drug.
Our goal was to look into specific characteristics of the drugs,
to see if they could predict if a registry was available.
We looked into drug characteristics.
For example, the level of innovation.
This is an algorithm based on the availability of a treatment
for a certain disease or the therapeutic effect.
And we looked into procedural characteristics, like:
does a drug have an orphan status?
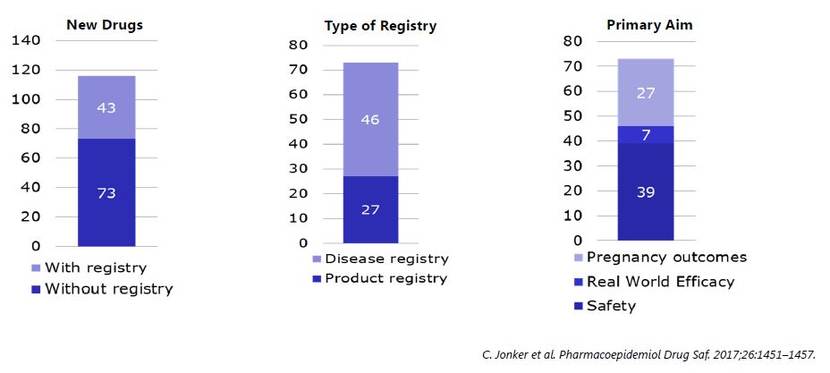
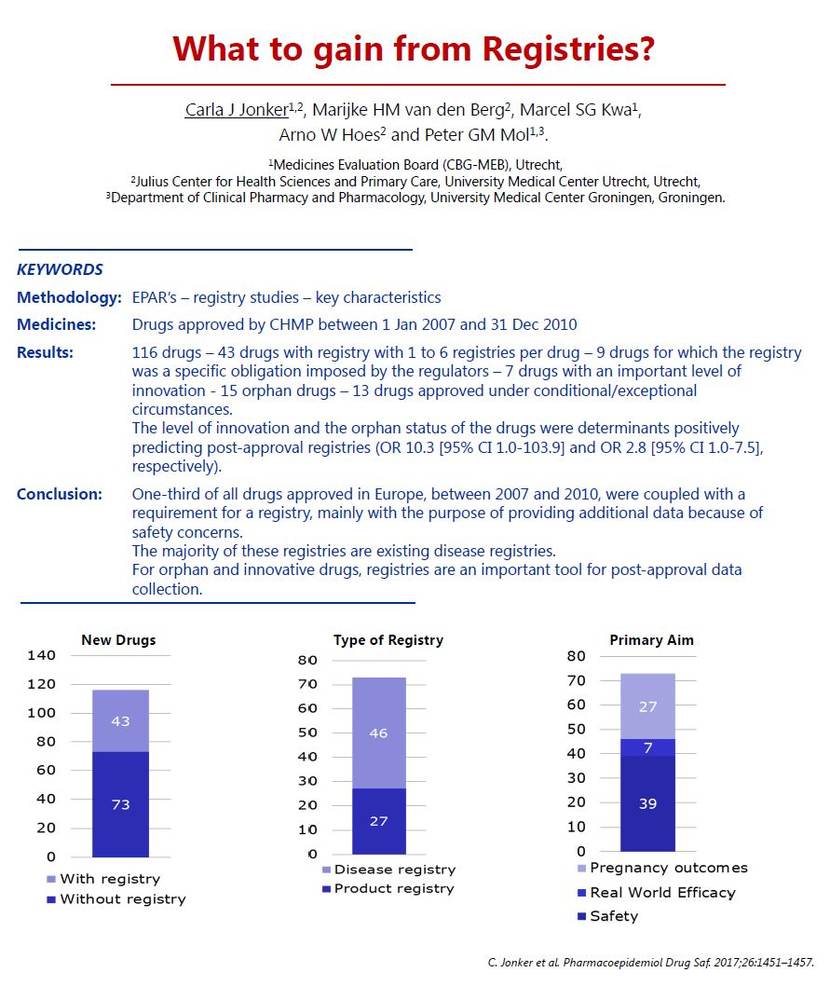
In my study, we found that there were 160 drugs approved,
and for one-third of them, a registry was requested.
It was one up to six.
So, we obtained a total of 73 registries.
Two-thirds of them were disease-based, and one-third, product registries.
The main reason for requesting a registry was because of safety,
for example to follow up on a safety concern,
or to collect data on women who are pregnant,
and in some cases we would like to know the real-world efficacy data.
The level of innovation and the orphan status
were positively related to having a registry in a dossier.
So for innovative drugs and orphan drugs,
a registry is important to collect data, post-approval.
(The MEB's logo, next to: Medicines Evaluation Board.)
For orphan and innovative drugs, registries are an important tool for data collection post‐approval.