Regulatory science provides the Medicines Evaluation Board (MEB) with up-to-date data, knowledge and expertise based on current scientific insights, for optimal evaluation and marketing authorisation of medicinal products and novel foods.
The studies that support the MEB's regulatory process are very diverse. The MEB Regulatory Science programme presents several studies as a Poster Pitch.
Poster pitch by D.J. Postma
Doerine Postma presents her study on medicine shortages.
(The MEB logo, next to: Poster Pitch by D.J. Postma: Medicine shortages.)
SILENCE
The motivation for this study was
that medicine shortages have an impact on patients.
Patients can have to switch to another label,
to another active ingredient or the active ingredient is totally absent.
Most studies are now based on surveys or on anecdotes, but not on hard data,
and this study is.
And by determining risk factors and understanding medicine shortages,
we can help to lessen the impact for patients.
The results, up until now, are based on one data set.
It is based on the reporting system from the Royal Dutch Pharmacists Association,
where pharmacies voluntarily report their shortages.
We're going to have a closer look at the data from authorities
where the marketing authorisation holders mandatorily report their shortages.
The results are now mainly based on observations from practice.
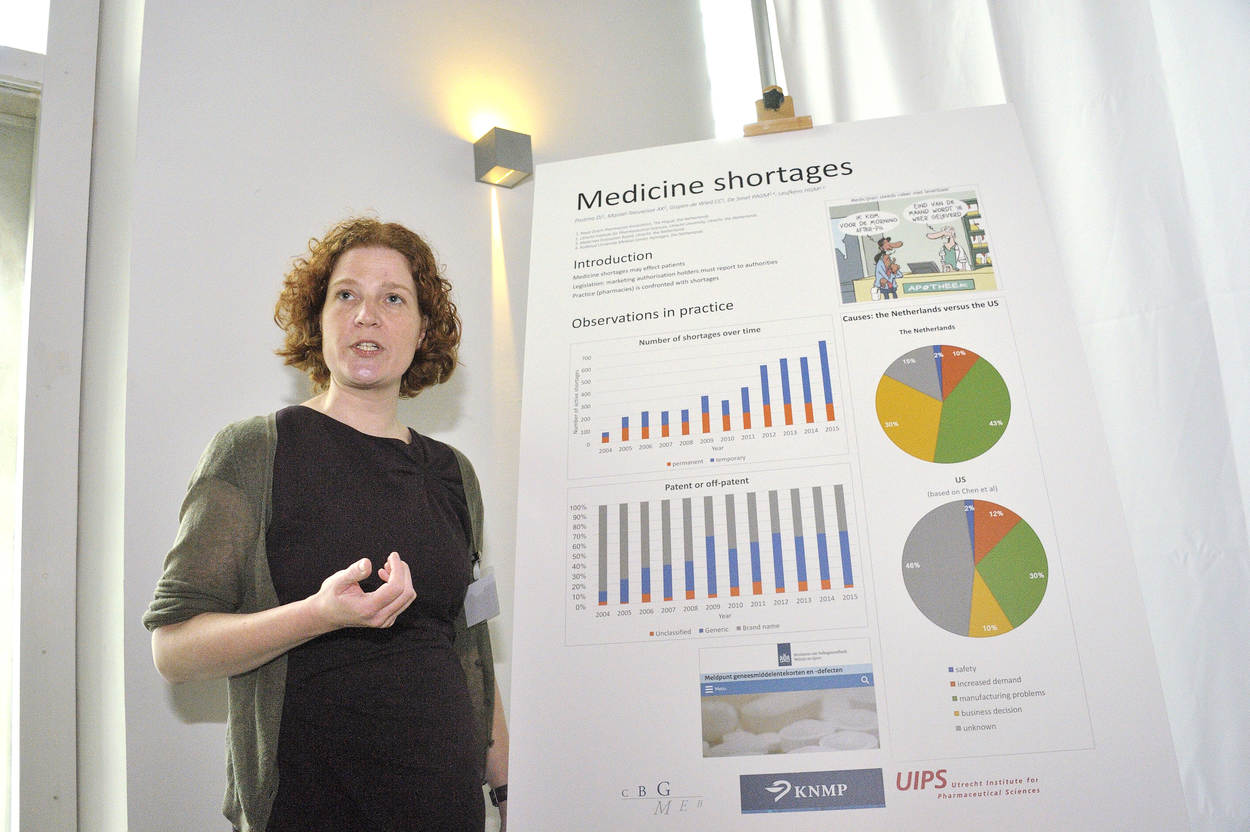
When we look at the number of shortages,
we see an increase especially in temporary shortages.
The number of permanent shortages is stable.
Looking at the difference between patented and off-patented medicine,
we see an increase of the patented medicine up until 2009,
and then the share of patented and off-patented medicine
is about 50 percent each.
Looking at the causes of medicine shortages,
we compared our results to the U.S.
And we see a difference in manufacturing problems,
and also a difference in business decisions.
It is hard to draw hard conclusions,
because a very large part of the causes are unknown in the US.
Since 1 January 2017,
the Medicine shortages and defects notification centre has been introduced.
Marketing authorisation holders have to report their shortages to the authority,
leading to sooner awareness of the shortages in practice.
(The MEB logo appears on a white background.)