Regulatory science provides the Medicines Evaluation Board (MEB) with up-to-date data, knowledge and expertise based on current scientific insights, for optimal evaluation and marketing authorisation of medicinal products and novel foods.
The studies that support the MEB's regulatory process are very diverse. The MEB Regulatory Science programme presents several studies as a Poster Pitch.
Poster pitch by P.J. Glerum
Pieter Glerum presents his study on "Robustness of the conclusion of bioequivalence; a non-parametric comparison."
(The MEB logo, next to: Poster Pitch by P.J. Glerum: Robustness of the conclusion of bioequivalence; a non-parametric comparison.)
SILENCE
We're performing this research because there is a lot of public debate
about efficacy, safety and quality of generic medicines.
And for the registration of generic medicines,
bioequivalence needs to be proven.
And we are challenging the concept of bioequivalence.
Is that still the best way to register generics?
The research is still ongoing. We're not sure yet what this means for the patient.
Basically, there are two possible outcomes for this research.
First would be that this supports the current policy and regulations
for the registration of generics.
Second would be that we would indicate some flaws
in the system and regulatory requirements.
So then, we're challenged to look at improvements of the system.
Either way, the patient or the healthcare professional can be ensured
that we challenge the concept of bioequivalence and make sure
that the best possible way of registration of generics is in practice.
To get to these results, uh...
together with University Maastricht and Radboud University,
we started a collaboration with the University of Southern California
to learn about pharmacokinetic modelling.
And we applied this mathematical modelling to a bioequivalence study
with four different formulations of Gabapentin,
of which one is the originator, and there are three generics.
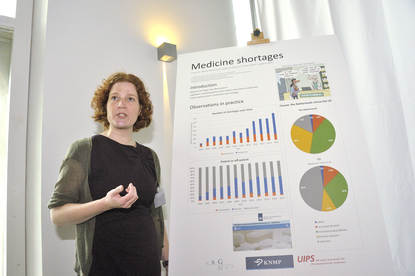
Now, if we look at the second picture on the poster,
this picture represents actually
the estimated absorption constant values per subject, colour-coded per formulation.
So, if this were the result of the research, there would actually be
an altered absorption profile estimated for one of the formulations.
But again, this is preliminary research, and the research is still ongoing.
(The MEB logo appears on a white background.)