RSNN workshop ‘Beyond the current clinical endpoints’
The RSNN had the Regulatory Science Network Netherlands workshop ‘Beyond the Current Clinical Endpoints’ on the 6th of July 2017. The Workshop was opened by Bert Leufkens, in his role as Chairman of RSNN.
There were four Keynote Speakers:
- Prof. Dr. Kit Roes (Julius Center/UMCU/MEB)
- Dr. Violeta Stoyanova (COMPEMA/MEB)
- Henk Kamsteeg (Janssen Biologics)
- Dr. Jacoline Bouvy (NICE), who shared her thoughts from an Health Technology Assessment (HTA) perspective.
During dinner, discussions continued. Marc Kaptein, medical director at Pfizer and Yvonne Schuller, researcher at AMC/UvA, accepted the invitation to take the stage.


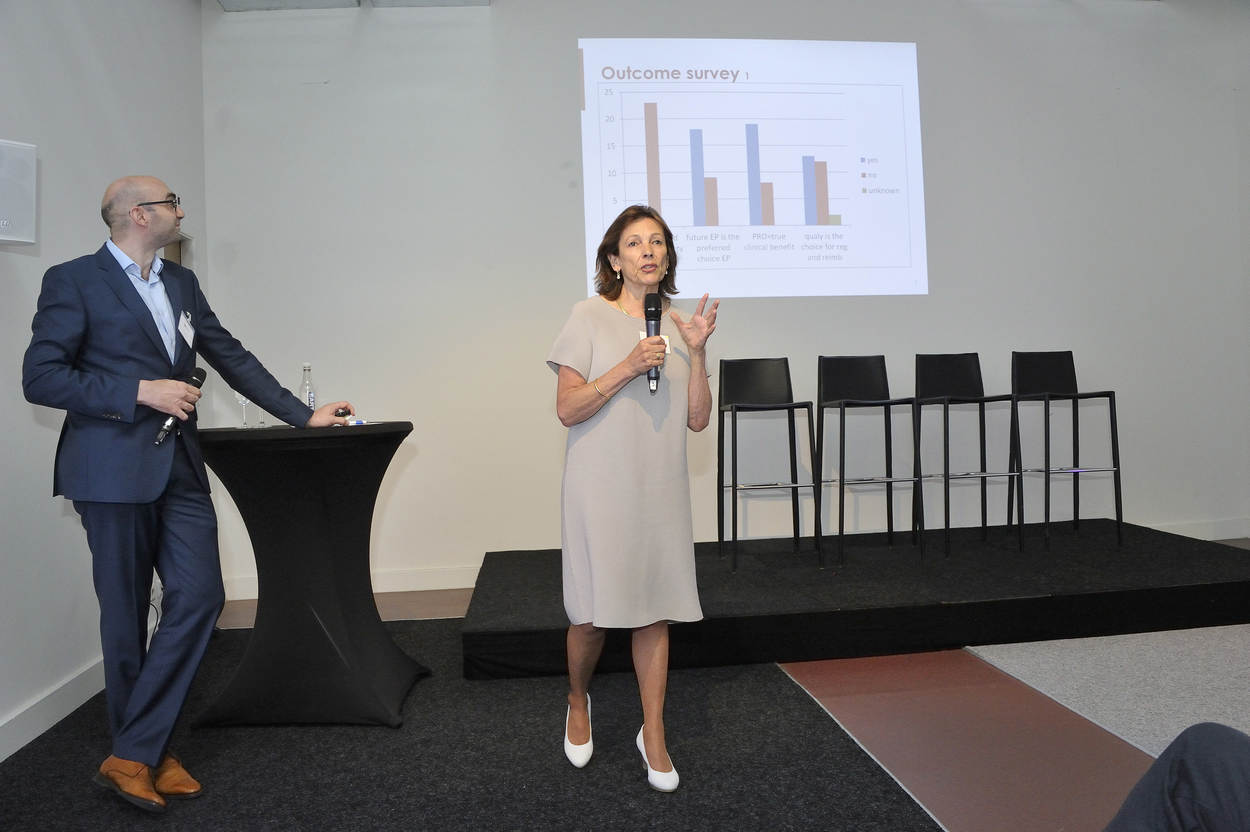
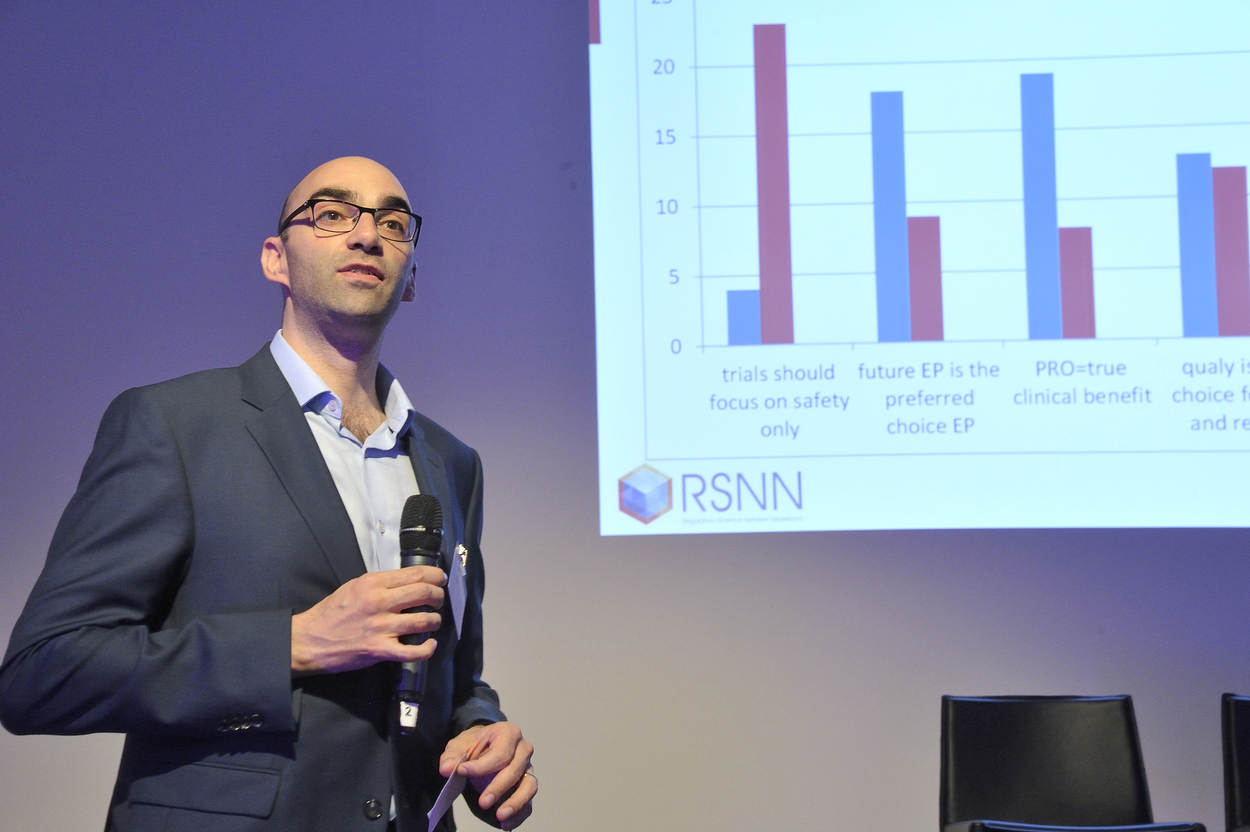
How can we improve the selection and use of challenging endpoints such as the ‘established’ 6-minute walk test (6MWT) or the less well-known ones such as the Goal Attainment Scaling (GAS)?
The majority of current clinical endpoints used in clinical trials can quantitatively inform us about the efficacy and clinical relevance of a new drug compared to a placebo or comparator in a randomized clinical trial. There are also endpoints that are difficult to translate into a clinically meaningful benefit. The reason for selecting endpoints that are not equal to a direct observable clinical event are diverse: endpoints may be chosen to be efficient, in case the clinically meaningful events are rare (accepted surrogate endpoints), they may be chosen as the best available validated standard for a diversity of patients, or are an accepted standard across treatments so that results can be compared.
For example, the six minute walk test (6MWT) is widely used as an endpoint to estimate effectiveness of interventions in numerous diseases such as osteoarthritis, and fibromyalgia, heart failure, chronic obstructive pulmonary disease (COPD), pulmonary fibrosis, pulmonary arterial hypertension (PAH), Duchenne Muscular Dystrophy, Fabry’s Disease, etc.. Reasons for use vary across diseases: as surrogate for the clinical outcome, as accepted standard or “the best we have”.
The popularity of the 6MWT lies partly in the fact that it is cost-effective, easy to perform and reproducible. The 6MWT has long been used, and with considerable success, as a functional endpoint to measure cardiovascular fitness and improvement. Conversely, it is also being used as a surrogate endpoint translating improvements in gained distance into potentially clinically relevant gains in quality of life for very complex neurodegenerative diseases. The use in neurodegenerative diseases has sparked a lot of discussion since it can be questioned whether distance can readily be translated to the most relevant complex functional outcomes.


More information
- More information about the RSNN Workshop you can read in the 3rd newsletter from the Regulatory Science Network Netherlands (RSNN). Read them here and become a member of the network.
- See also Lygature Partnerships MeetUp 2017, 12 October 2017.
-
Take a fine look at our photo impression of the RSNN Workshop.