Regulatory science provides the Medicines Evaluation Board (MEB) with up-to-date data, knowledge and expertise based on current scientific insights, for optimal evaluation and marketing authorisation of medicinal products and novel foods.
The studies that support the MEB's regulatory process are very diverse. The MEB Regulatory Science programme presents several studies as a Poster Pitch and also some Portraits.
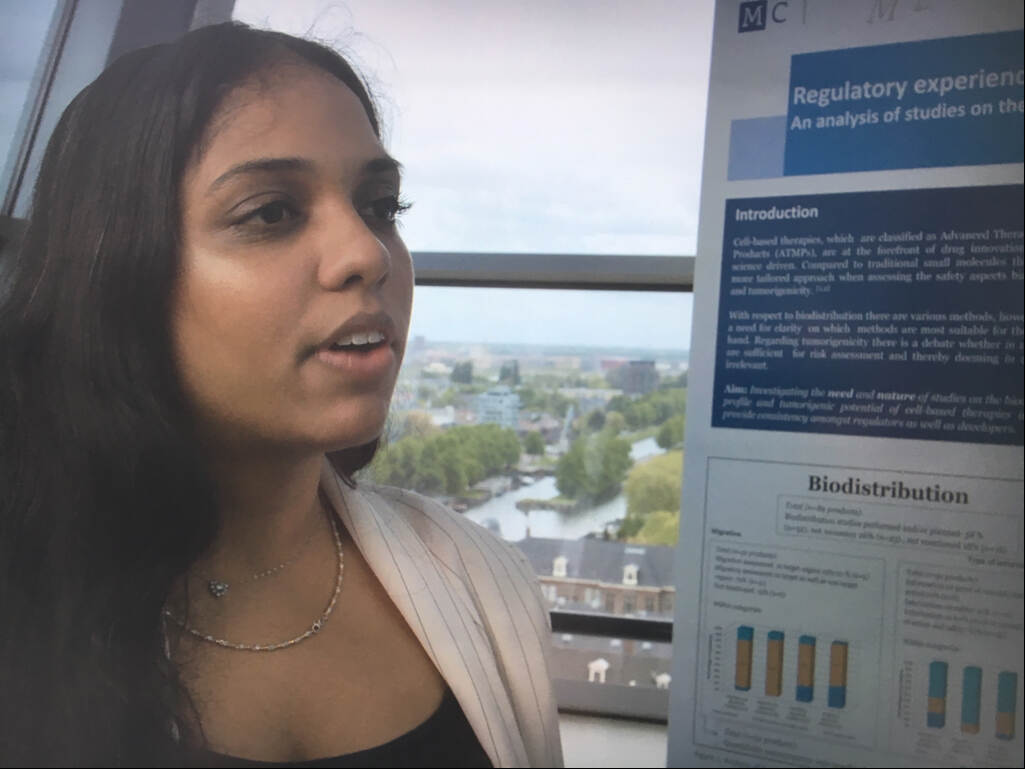
Title: Poster Pitch by T.J. Nakchedi
Regulatory experience with non-clinical studies of cell-based therapies.
My name is Tahira Nakchedi and I’m in my final year of the master Pharmacy. I performed researched at the MEB on the biodistribution profile and
tumorigenic potential of cell-based therapies. The focus of my research was on cell-based therapies, which are all products that belong to the class of advanced therapy medicinal products consisting of cells. When developing these cell-based therapies, a tailored approach is required for the biodistribution profile and tumorigenic potential.
When it comes to the biodistribution profile, there are various techniques that can be used to measure the biodistribution profile. Whilst for tumorigenicity there is a debate on the relevance of animal studies versus the sufficiency of in vitro methods. This can be challenging for companies developing these products, but it is also challenging for regulators when providing scientific advice when companies request this. Therefore, the aim of my research was to provide insight into the need for and the nature of biodistribution and tumorigenicity studies, to provide consistency amongst companies as well as regulators.
For the purpose of this research, data was collected from scientific advice reports within between 2015 and mid 2018. A total of 89 products were selected and sorted into a score table. For further analysis the products were sorted into four categories which are: autologous not genetically modified, allogeneic not genetically modified, autologous genetically modified and allogeneic genetically modified products.
Moving over to the results it can be seen that for biodistribution the need for studies is high. As this is the case for 58% of products. When looking at the nature of biodistribution studies, it is recognized by companies that it is important to measure the migration to target as well as non-target organs as this was the case for 79% of products. When looking at the type of information that can be acquired from these biodistribution studies it is usually a proof of concept as well as safety. Additionally, it is useful when quantifiable measurements can be acquired, which was the case for 83% of products.
As mentioned before, there are various techniques that can be used to measure the biodistribution. Therefore, I performed a systematic literature search, to provide an overview table that contains parameters such as: sensitivity, resolution, imaging speed/time frame which can facilitate in the decision for companies and regulators in which technique is most suitable for the product being developed.
When looking at the other aspect of this research, tumorigenicity, it is apparent that tumorigenicity is studied in vitro as well as in vivo for most products, which is around 42% of products. When looking at the relevance of the performed in vivo studies with regards to the aforementioned debate, the data shows that for 83% of products the animal studies performed, were not regarded as relevant. Keeping in mind that in vitro data was available for 63% of products and the fact that for half of the products the tumorigenicity data is regarded as fully informative, suggest that the in vitro data can be sufficient to determine the tumorigenic potential deeming in vivo studies unnecessary.
Poster Tahira Nakchedi: Regulatory experience with non-clinical studies of cell-based therapy (PDF)